¶ Experimental Techniques used in Condensate Research
A wide variety of techniques are used in condensate research depending on purpose. This page aims to briefly summarise the purpose of these experiments within the condensate context; for further details a primer by Alberti et al. is a good place to start[1].
¶ Microscopy
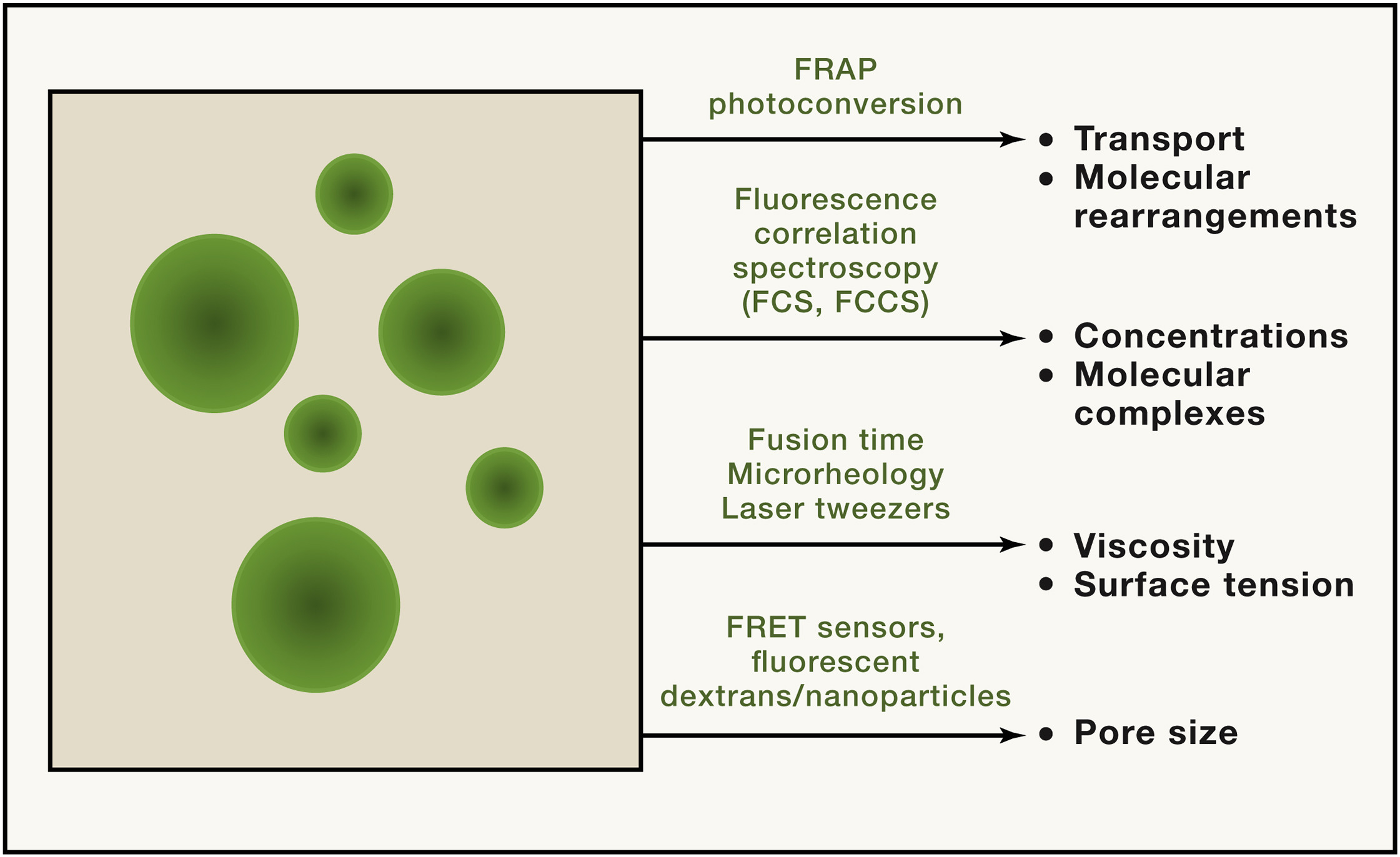
Different aspects of condensate function and behaviour can be quantified.[1:1]
A wide variety of microscopy techniques exist to investigate condensate formation, morphology and content.
¶ Light Microsopy
Timelapsed Light microscopy can be used to investigate LLPS-induced condensate formation. Induction of a change in condition (e.g. addition of nucleic acid, alteration of pH/salt concentrations) can lead to condensate formation from a homogeneous solution and detected by conventional light microscopy. Experimental parameters including incubation time should be monitored and kept constant. Interaction with the glass surface of the microscope slide may induce artifacts in the material properties via dewetting and so coating of the slide with suitable lipids/PEG is recommended. [1:2]
¶ Fluorescent Microscopy
Fluorescent microscopy forms the basis of many experimental techniques involved in condensate formation. As a standalone technique it is effective in verifying if a particular fluorophore-tagged molecule partitions into the condensate and for (co-)localisation studies. Fluorescent labelling of nucleotides is also possible to study nucleic acid recruitment into droplets, making fluorescent microscopy a versatile tool in studying all types of condensate constituents. [1:3]
¶ Fluorescent Recovery After Photobleaching (FRAP)
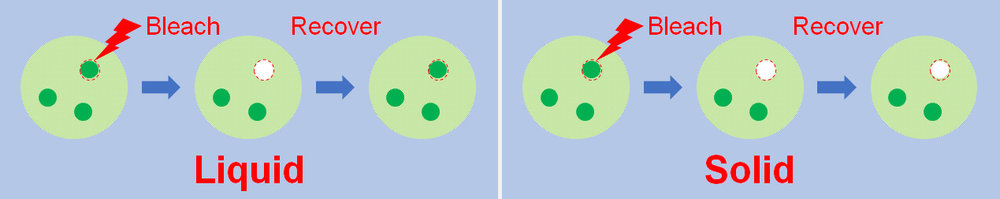
FRAP can be used to determine the material properties of the condensate (such gel-like properties). Recovery of fluorescence in more solid-like, viscous condensates following laser bleaching will be slower than in more liquid-like condensates. Credit to the Ke Zhang lab. [2]
FRAP experiments are widely used to ascertain the liquid-like properties of a condensate and can be used to measure diffusion coefficients within condensates[3]. On its own, however, they are inconclusive in showing that a condensate necessarily forms from LLPS [4].
¶ Mass spectrometry (MS)
Mass spectrometry is a class of techniques which allows for the high-throughput identification of proteins in a condensate[5][6]. However, mass spectrometry lacks information as to the localisation of the protein within the condensate, whilst the isolation techniques may also affect the list of candidate protein constituents identified[6:1].
¶ Fluorescence Correlation Spectroscopy (FCS)
A sensitive technique using statistical analysis to study stationary fluctuations of the fluorescence intensity [7]. FCS can also be used to measure the kinetic properties of the condensate, especially diffusion constants, which can provide insights into the material properties of the condensate.
Alberti et al. (2019), Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. ↩︎ ↩︎ ↩︎ ↩︎
Taylor et al. (2019). Quantifying Dynamics in Phase-Separated Condensates Using Fluorescence Recovery after Photobleaching ↩︎
Lyon et al. (2020) A framework for understanding the dunctions of biomolecular condensates across scales ↩︎
J.S. Andersen, Y.W. Lam, A.K.L. Leung, S.-E. Ong, C.E. Lyon, A.I. Lamond, M. Mann, Nucleolar proteome dynamics, Nature 433 (2005) 77. ↩︎
Ditlev, J. A., Case, L. B. & Rosen, M. K. Who’s In and Who’s Out—Compositional Control of Biomolecular Condensates. Journal of Molecular Biology 430, 4666–4684 (2018). ↩︎ ↩︎
https://en.wikipedia.org/wiki/Fluorescence_correlation_spectroscopy ↩︎