¶ Scaffolds and Clients
In biological condensates, the scaffold and client nomenclature is used to discern the differing roles of their constituents in promoting the formation of the condensate. The scaffold-client definition applies not only to proteins but to nucleic acids as well.
A scaffold is defined by its necessity in promoting the formation of the particular condensate [1]. A scaffold is usually multivalent, thus facilitating a wide array of interactions with multiple partners, collectively with high-avidity[2]. Consequently, scaffold proteins invariable contain intrinsically disordered regions (IDRs) that provide multivalent binding sites [3][4].
A client is selectively recruited into the condensate, often via interaction with scaffolds but is itself not necessary nor sufficient in driving the formation of the condensate [1:1][5].
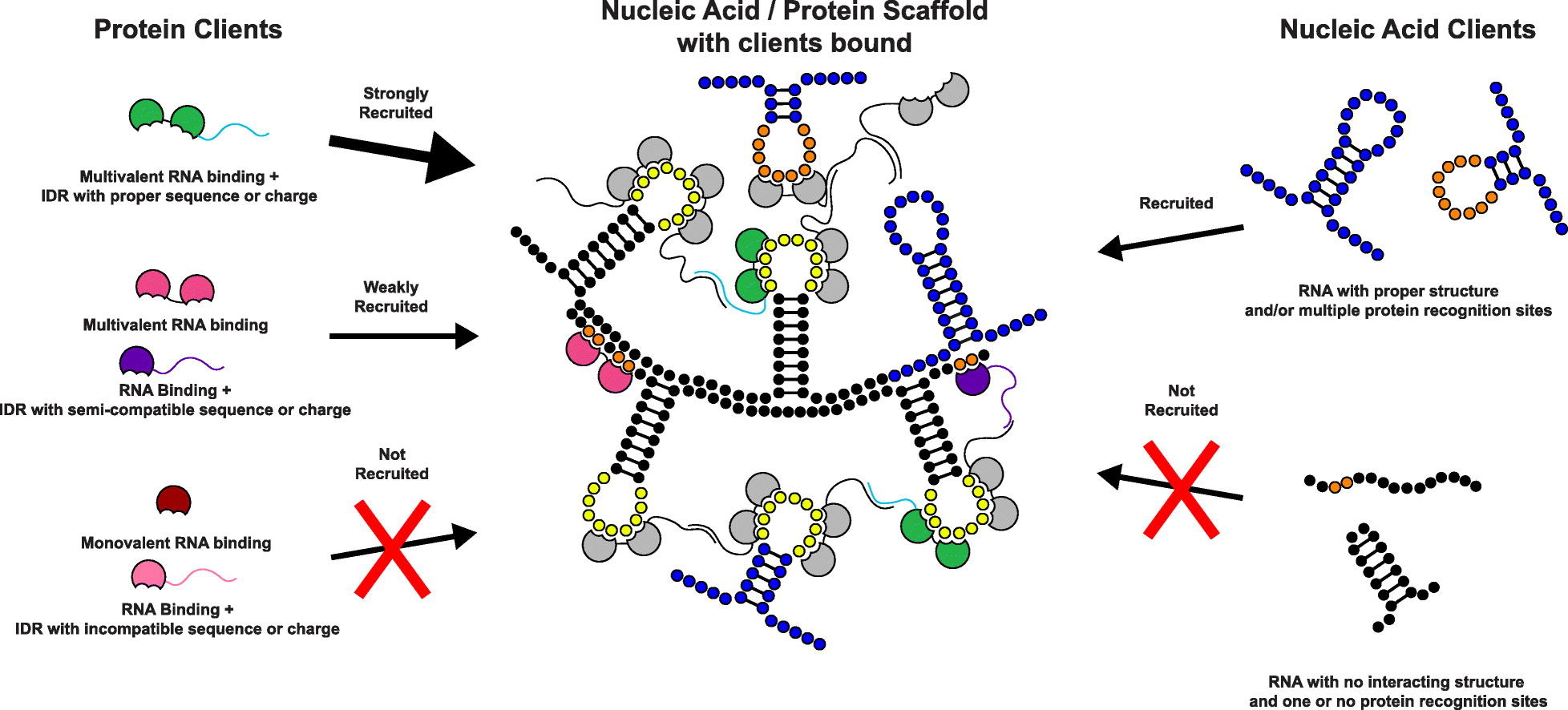 A diagram of the scaffold-client model showing the types of interactions that can drive client recruitment into a condensate.[5:1]
A diagram of the scaffold-client model showing the types of interactions that can drive client recruitment into a condensate.[5:1]
Whereas the definitions suggest a binary classification, in natural condensates the scaffold-client model exists as a spectrum in which the constituents lie [1:2]. Furthermore, the designation of a particular constituent as a scaffold/client is only relevant with respect to a particular condensate, i.e. a protein can be a client in one condensate but a scaffold in another, as was shown for the FUS protein [5:2].
We have redefined the proteins on CD-CODE and divided them into two groups, drivers and members.
Drivers: Proteins which have at least one of the following features.
Induce the formation of a condensate.
Are essential for the integrity of a condensate.
Clients: Proteins which are part of a condensate, but driven into it by driver proteins.
Lyon et al. (2020). A framework for understanding the functions of biomolecular condensates across scales. ↩︎ ↩︎ ↩︎
Posey, A. E., Holehouse, A. S. & Pappu, R. V. Phase Separation of Intrinsically Disordered Proteins. in Methods in Enzymology vol. 611 1–30 (Academic Press Inc., 2018). ↩︎
Boeynaems, S. et al. Protein Phase Separation: A New Phase in Cell Biology. Trends in Cell Biology vol. 28 420–435 (2018). ↩︎
Uversky, V. N. Intrinsically disordered proteins and their ‘Mysterious’ (meta)physics. Frontiers in Physics vol. 7 (2019). ↩︎
Ditlev, J. A., Case, L. B. & Rosen, M. K. Who’s In and Who’s Out—Compositional Control of Biomolecular Condensates. Journal of Molecular Biology 430, 4666–4684 (2018). ↩︎ ↩︎ ↩︎