¶ Mechanisms of Condensate Formation
Biological condensates can form either via energy-consuming (active) or thermodynamically passive mechanisms. The former requires energy expenditure whilst the other is a thermodynamically spontaneous process. However, these mechanisms are not mutually exclusive; for example, both active and passive processes drive the formation of the nucleolus [1].
¶ Liquid-liquid phase separation (LLPS)
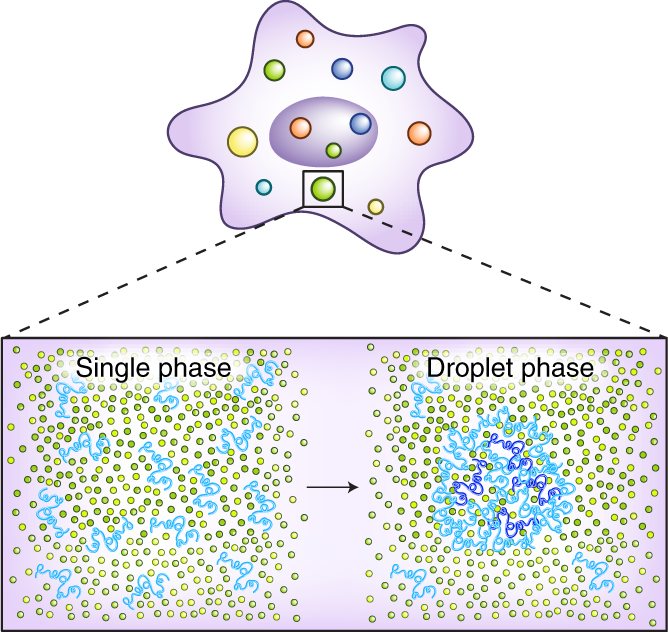
A simple diagram showing the transition from a homogeneous (monophasic) solution to the appearance of phase separated condensates via a phase transition[2]
LLPS is one of the many thermodynamically spontaneous/passive procceses by which condensates can be formed. In LLPS, the transition between the one-phase (homogeneous) and the two-phase regime (phase-separated) is infinitely cooperative at the saturation concentration (Csat or critical concentration, Ccrit) [3]. The Csat is the concentration of the solute (protein/nucleic acid) at which it separates into a solute-depleted dilute phase and a solute-enriched dense phase. Csat is heavily influenced by environmental conditions such as temperature, ionic strength and pH, whilst post-translational modifications such as phosphorylation, SUMOylation can drastically influence the Csat of the native protein [3:1]. Condensates formed by LLPS also exhibit interfacial tension between the 2 phases leading to a spherical morphology to mimimise surface area [1:1]